How to Calculate Empirical Formula
Molecular formula C 3 H 6. Calculating an empirical formula Information about reacting masses is used to calculate empirical formulae.

Empirical And Molecular Formula Notes Chemistry Worksheets Teaching Chemistry Chemistry Notes
Start by converting the mass of each.

. Round it off if needed. Multiply the numbers in the empirical formula by the factor 3. Hooray for no more confusionCheck out my NEW complete guide on Empir.
We were given the molecular weight of the molecule 18018 gmol. This is obtained from experiments. Divide this number by the molecular weight of the empirical formula to find the number of empirical.
Example A hydrocarbon contains 48 g of carbon. This Empirical Formula Calculator finds an empirical formula corresponding to the given compound chemical composition. Enter in the corresponding fields of the calculator the.
Also our online empirical formula. Generally speaking in empirical formula problems C 12 H 1 O 16 and S 32 are sufficient. If you hit a problem that just.
How to calculate empirical formula An empirical formula can be calculated through chemical stoichiometry. The empirical formula for a compound is C 2 H 5 and its relative formula mass is 58. The algorithm below explains how to use the empirical rule.
The mass of the atoms in the empirical. The result will be a whole number or close to a whole number. If we know which elements are present in a molecule and in what ratio we can.
Add up the atomic masses of the atoms in the empirical formula. N - the number of. So the simplest formula of the compound is CH2O.
X i - each individual value from your data. Determine the masses of each component in the compound. Find the empirical formula of the compound.
Divide given molar mass by empirical formula mass. Converting empirical formulae to molecular formulae. μ Σ x i n.
There are times when using 12011 or 1008 will be necessary. This video goes into detailed steps on how to find the empirical formula of a compound. Calculate the empirical formula mass.
Calculate the mean of your values. Determine the number of moles by dividing the grams by the atomic mass. You can work out the molecular formula from the empirical formula if you know the relative mass formula M r of the compound.
Formula to calculate empirical formula. For example the empirical formula of a hydrocarbon is CH2 and its Mr is 42. Find the simplest formula.
A compound is analyzed and calculated to consist of 135 g Ca 108 g O and 0675 g H.

Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular
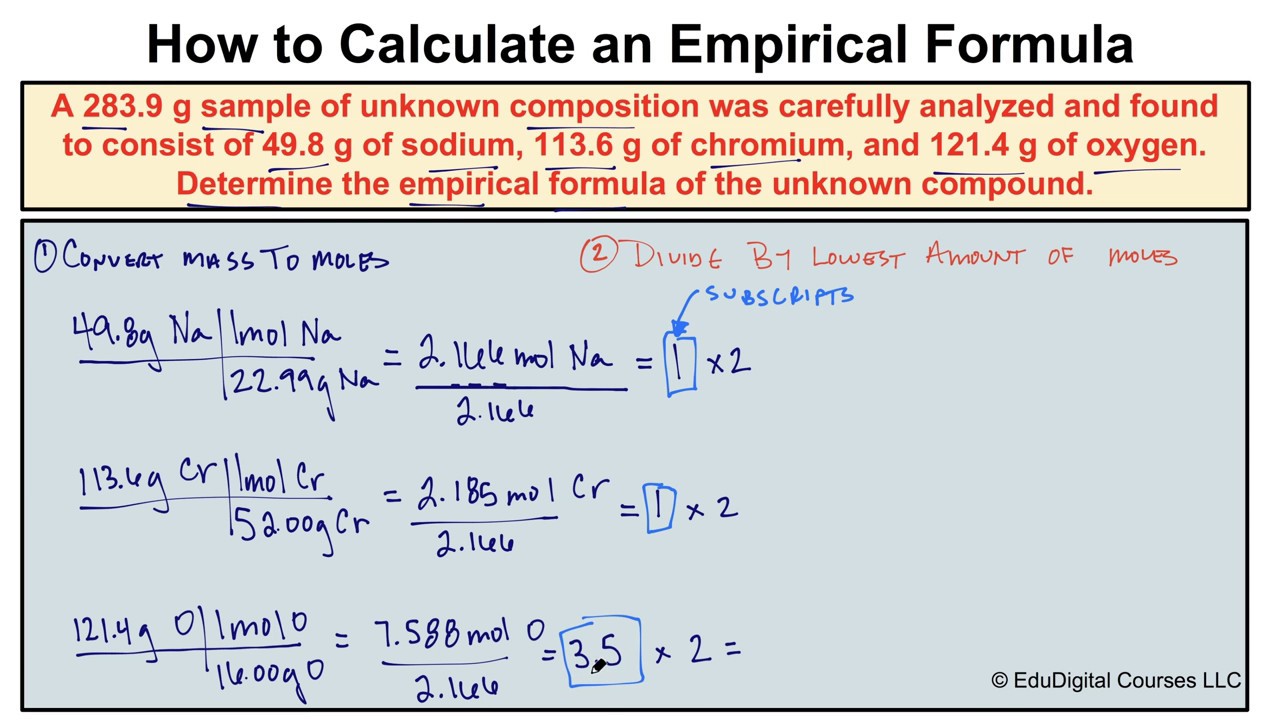
How To Calculate An Empirical Formula Chemistry Worksheets Chemistry Notes Scientific Method Worksheet

Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular
No comments for "How to Calculate Empirical Formula"
Post a Comment